Alpha Particle Scattering Experiment-
On the suggestion of Rutherford, his two associates Geiger and Marsden in 1911 performed an experiment on the scattering of alpha particles and obtained an important insight into the structure of the atom.
This led to the birth of the nuclear model of an atom– a major step towards how we see the atom today.
Experimental Arrangement-
The following diagram shows the schematic arrangement of the Geiger-Marsden experiment-
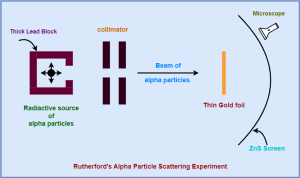
The above experimental arrangement is explained in the following steps-
Step-01:
- A radioactive source of alpha particles is enclosed in a thick lead block provided with a narrow opening.
- Lead block is used to keep the source because lead has high density and alpha particles cannot penetrate through it.
Step-02:
- The alpha particles from this source are collimated (make rays of particles accurately parallel) into a narrow beam through a narrow slit.
Step-03:
- The beam is then allowed to fall on a thin gold foil.
- For the scattering experiment, Rutherford wanted a metal sheet which could be as thin as possible.
- Gold is the most malleable of all known metals. So, it could be easily converted into a very thin sheet.
- Hence, Rutherford selected a gold foil for his alpha particle scattering experiment.
Step-04:
- After hitting the gold foil, the alpha particles scatter in different directions.
- The alpha particles scattered in different directions are observed with the help of a rotatable detector which consists of a zinc sulphide screen and a microscope.
- Zinc sulphide is a scintillating compound. Zinc sulphide screen has a unique quality whenever a charged particle hits the screen, the spot at which the particle hits, glows for sometime.
- This allows to study the scattering of alpha particles by the brief flashes on the screen.
- The spot is viewed through the microscope. In this way, the number of alpha particles scattered at different angles are counted.
Step-05:
- The whole apparatus is enclosed in an evacuated chamber to avoid the scattering of alpha particles by air molecules.
Observations-
The following observations were made-
- Most of the alpha particles (about 99.86%) pass straight through the gold foil.
- A few alpha particles (about 0.14%) deviate through small angles (around 1° or more).
- A very few alpha particles about 1 in 8000 get deflected through 90° or more.
- A very-very few alpha particles about 1 in 20000 get rebounded from the gold foil suffering a deflection of nearly 180°.
Conclusions-
The following facts were concluded about an atom-
- As most of the alpha particles pass straight through the foil, so most of the space within atoms must be empty.
- As few alpha particles deviate through small angles, so there must be some positive charge in the atom.
- To explain the large scale scattering of alpha particles, Rutherford suggested that all the positive charge and the mass of the atom is concentrated in a very small region called the nucleus of the atom.
- The nucleus is surrounded by a cloud of electrons whose total negative charge is equal to the total positive charge on the nucleus so that the atom as a whole is electrically neutral.
Read the next article on-
Get more notes & other study material of the Chapter Atoms.

