Spectral Series of Hydrogen Atom-
Before you go through this article, make sure that you have gone through the previous article on Bohr’s Atomic Model.
We have learnt that-
- Electrons revolve around the nucleus in fixed energy orbits called as stationary states.
- An electron does not absorb or radiate energy while moving in these stationary states.
In this article, we will discuss about spectral series of hydrogen atom.
Spectral Series of Hydrogen Atom-
From Bohr’s theory, the energy of an electron in nth Bohr orbit of hydrogen atom is given by-
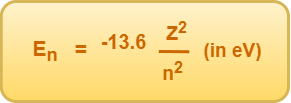
(For hydrogen atom, Z = 1)
According to Bohr’s frequency condition, whenever an electron makes a transition from a higher energy level n2 to a lower energy level n1, the difference of energy appears in the form of a photon. The frequency ν of the emitted photon is given by-

Wavelength of Emitted Photon-
The wavelength of the emitted photon is given by-

This formula indicates that the radiation emitted by the excited hydrogen atom consists of certain specific wavelengths or frequencies, the value of which depend on quantum numbers n1 and n2.
Wave Number-
Wave number is defined as the reciprocal of wavelength λ. It is given by-
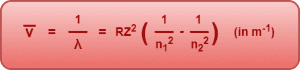
Spectral Series of Hydrogen Atom-
The origin of the various series in the hydrogen spectrum can be explained as follows-
1. Lyman Series-
- If an electron jumps from any higher energy level n2 = 2, 3, 4, …… to a lower energy level n1 = 1, we get a set of spectral lines called as Lyman series.
- It belongs to the ultraviolet region of the electromagnetic spectrum.
This series is given by-
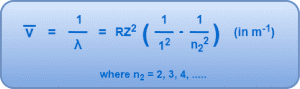
2. Balmer Series-
- If an electron jumps from any higher energy level n2 = 3, 4, 5, …… to a lower energy level n1 = 2, we get a set of spectral lines called as Balmer series.
- It belongs to the visible region of the electromagnetic spectrum.
This series is given by-

3. Paschen Series-
- If an electron jumps from any higher energy level n2 = 4, 5, 6, …… to a lower energy level n1 = 3, we get a set of spectral lines called as Paschen series.
- It belongs to the infrared region of the electromagnetic spectrum.
This series is given by-
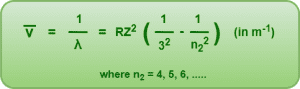
4. Brackett Series-
- If an electron jumps from any higher energy level n2 = 5, 6, 7, …… to a lower energy level n1 = 4, we get a set of spectral lines called as Brackett series.
- It belongs to the infrared region of the electromagnetic spectrum.
This series is given by-
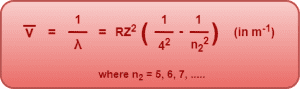
5. Pfund Series-
- If an electron jumps from any higher energy level n2 = 6, 7, 8, …… to a lower energy level n1 = 5, we get a set of spectral lines called as Pfund series.
- It belongs to the infrared region of the electromagnetic spectrum.
This series is given by-
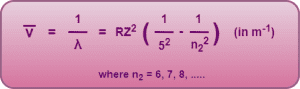
Shortest and Longest Wavelength of a Spectral Series-
We know, if an electron makes a transition from any higher energy level n2 to any lower energy level n1, then the wavelength of the photon emitted during transition is given by-
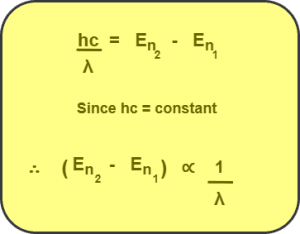
From here, we conclude that the wavelength of the emitted photon is smallest when the energy difference between the two levels is largest and vice-versa.
RememberFor smallest wavelength, consider the longest transition For longest wavelength, consider the smallest transition |
We can summarize the concept of longest and shortest wavelength of spectral series in the following table-
| Series | Longest wavelength occur during transition | Shortest wavelength occur during transition |
| Lyman | n2 = 2 to n1 = 1 | n2 = ∞ to n1 = 1 |
| Balmer | n2 = 3 to n1 = 2 | n2 = ∞ to n1 = 2 |
| Paschen | n2 = 4 to n1 = 3 | n2 = ∞ to n1 = 3 |
| Brackett | n2 = 5 to n1 = 4 | n2 = ∞ to n1 = 4 |
| Pfund | n2 = 6 to n1 = 5 | n2 = ∞ to n1 = 5 |
Read the next article on-
Energy Level Diagram For Hydrogen Atom
Get more notes & other study material of the Chapter Atoms.

