Rutherford’s Atomic Model-
Before you go through this article, make sure that you have gone through the previous article on Rutherford’s Atomic Model.
We have learnt that-
- With alpha particle scattering experiment, Rutherford led to the birth of nuclear model of an atom in 1911.
- But the Rutherford’s atomic model suffered from certain limitations.
- Rutherford’s atomic model could not explain the stability of an atom.
- Rutherford’s atomic model also could not explain the observed line spectrum.
In this article, we will discuss about Bohr’s Atomic Model.
Bohr’s Atomic Model-
- Bohr’s Atomic Model was proposed by Neil Bohr in 1913.
- Rutherford basically explained the nucleus of an atom and Bohr modified that model into electrons and their energy levels.
Postulates of Bohr’s Atomic Model-
Bohr’s atomic model is based upon the following postulates-
Postulate-01:
An atom consists of a small and massive central core called the nucleus around which electrons revolve in definite circular paths called as orbits or shells.
The centripetal force required for their revolution is provided by the electrostatic attraction between the electrons and the nucleus.
Postulate-02:
Of all the possible circular orbits, the electrons are allowed to revolve only in those orbits in which the angular momentum of an electron is an integral multiple of h/2π, where h is a Planck’s constant.
Therefore, for any permitted orbit,

Bohr’s Quantization Condition of Angular Momentum
where-
- L = Angular momentum of the electron
- m = mass of the electron
- v = speed of the electron
- r = radius of the permitted orbit
- n = positive integer called principal quantum number
The above equation is Bohr’s famous quantum condition called Bohr’s quantization condition of angular momentum.
Postulate-03:
While revolving in the permissible orbits, an electron does not radiate energy. These non-radiating orbits are called as stationary states.
Postulate-04:
An atom can emit or absorb radiation in the form of discrete energy photons only when an electron jumps from a higher to a lower orbit or from a lower orbit to a higher orbit respectively.
If E1 and E2 are the energies associated with these permitted orbits, then the frequency v of the emitted or absorbed radiation is given by-
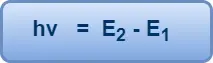
This is Bohr’s famous frequency condition.
Read the next article on-
Get more notes & other study material of the Chapter Atoms.

