Rutherford’s Alpha Particle Scattering Experiment-
Before you go through this article, make sure that you have gone through the previous article on Rutherford’s alpha particle scattering experiment.
We have learnt that-
- In alpha particle scattering experiment, the alpha particles were bombarded on thin gold foil.
- After the collision, the alpha particles were scattered in different directions.
In this article, we will learn about impact parameter.
Impact Parameter-
The impact parameter is defined as the perpendicular distance of the initial velocity vector of the alpha particle from the center of the nucleus when it is far away from the atom.
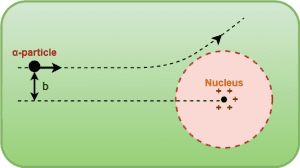
Here, ‘b’ denotes the impact parameter.
Significance of Impact Parameter-
The scattering of an α-particle from the nucleus depends on its distance of closest approach to the nucleus or an equivalent length called the impact parameter ‘b’.
From experiments, one can notice the following points-
For large impact parameter,
- The repulsive force experienced by the α-particle is weak.
- So, the α-particle passes almost undeflected.
For small impact parameter,
- The repulsive force experienced by the α-particle is large.
- So, the α-particle is scattered through large angle.
For impact parameter b = 0,
- The α-particle just aims at the center of the nucleus (head-on collision).
- So, the α-particle suffers a deflection of 180° and is reversed back along its original path.
Read the next article on-
Limitations of Rutherford’s Atomic Model
Get more notes & other study material of the Chapter Atoms.

