Alpha Particle Scattering Experiment
Before you go through this experiment, make sure that you have gone through the previous article on Rutherford’s α-particle scattering experiment.
We have discussed-
- On the suggestion of Rutherford, his two associates Geiger and Marsden in 1911 performed an experiment on the scattering of alpha particles.
- They obtained an important insight into the structure of the atom.
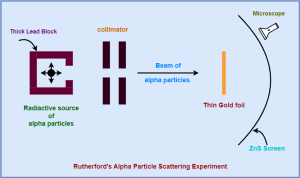
In this article, we will discuss Rutherford’s atomic model and its limitations.
Rutherford’s Atomic Model-
On the basis of alpha particle scattering experiment, Rutherford proposed the following model of an atom-
Postulate-01:
An atom consists of a small and massive central core in which the entire positive charge and almost the whole mass of the atom are concentrated. This core is called the nucleus.
Postulate-02:
The size of the nucleus (of the order 10-15 m) is very small as compared to the size of the atom (of the order 10-10 m).
Postulate-03:
The nucleus is surrounded by a suitable number of electrons so that their total negative charge is equal to the total positive charge on the nucleus and the atom as a whole is electrically neutral.
Postulate-04:
The electrons revolve around the nucleus in various orbits just as planets revolve around the sun. The centripetal force required for their revolution is provided by the electrostatic force of attraction between the electrons and the nucleus.
Read the next article on-
Get more notes & other study material of the Chapter Atoms.

