Electric Cell-
An electric cell may be defined as-
| An electric cell is a device which supplies energy to the charge carriers and thereby maintain their flow in the electric circuit. |
Working of Electric Cell-
- By using chemical reactions, a cell produces the potential difference across its terminals.
- When the terminals of cell are connected by a wire, the potential difference between the terminals of cell set up an electric field within the wire.
- Due to this electric field, the charge carriers experiences an electric force.
- This starts the flow of electric current around the loop.
Internal Resistance of Electric Cell-
The internal resistance of an electric cell may be defined as-
| The resistance offered by the electrolyte of a cell to the flow of current between its electrodes is called as internal resistance of electric cell. |
- The internal resistance is produced due to the collisions between the ions of the electrolyte.
- A freshly prepared cell has low internal resistance but its value increases as we draw more and more current from it.
- An ideal cell has zero internal resistance but all practical cells that we use have some internal resistance.
Representation of Electric Cell-
An ideal cell has zero internal resistance and is represented as-

Ideal Cell
A practical cell has some finite internal resistance and is represented as-

Practical Cell
EMF of Cell-
The emf of an electric cell may be defined as-
|
EMF of a cell is the potential difference measured across the cell when it is not connected to any external circuit.
OR EMF of a cell is the potential difference between its terminals in an open circuit i.e. when no current flows through the cell. |
Emf of a cell is denoted by the symbol E.
It is interesting to note that EMF stands for electromotive force but it does not really refer to a force rather it describes a potential difference in volts.
Consider an electric cell as shown-

Using open loop rule from A to B, we have-
VA – E = VB
VA – VB = E
Thus, when cell is not connected to any external circuit, potential difference across its terminals is equal to the emf of the cell.
Terminal Voltage of Cell-
The terminal voltage of an electric cell may be defined as-
|
Terminal voltage of a cell is the potential difference measured across the cell when it is connected to an external circuit.
OR Terminal voltage of a cell is the potential difference between its terminals in a closed circuit i.e. when current flows through the cell. |
Following two cases are possible-
Case-01: When current comes out of the positive terminal of cell (Discharging of Cell)-
Consider the following electric circuit-
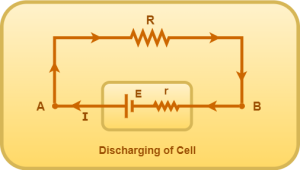
Here, current comes out of the positive terminal of the cell (E, r). In this case, the cell discharges.
Using open loop rule from A to B, we have-
VA – E + Ir = VB
VA – VB = E – Ir
Thus, during discharging of cell, terminal voltage across the cell = E – Ir.
Important Notes-
Note-01:
- During discharging, terminal voltage of cell is always less than its emf.
- This is because certain amount of voltage equal to Ir drops across its internal resistance (r).
Note-02:
- If the two ends of cell are connected using only a plane wire, then terminal voltage across the cell becomes zero.
- In this case, maximum current = E / r is drawn from the cell.
Case-02: When current goes into the positive terminal of cell (Charging of Cell)-
Consider the following electric circuit-
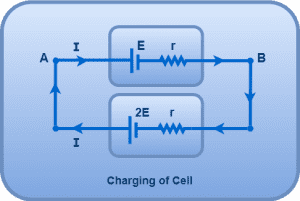
Here, current goes into the positive terminal of the cell (E, r). In this case, this cell charges.
Using open loop rule from A to B, we have-
VA – E – Ir = VB
VA – VB = E + Ir
Thus, during charging of cell, terminal voltage across the cell = E + Ir.
Important Notes-
Note-01:
- During charging, terminal voltage of cell is always greater than its emf.
Note-02:
- During charging, terminal voltage of cell cannot be zero.
Test Your Concepts-
Quiz on Electric Cell | EMF & Terminal Voltage
Next Article-
Get more notes & other study material of the Chapter Current Electricity.

