Resistance-
Before you go through this article, make sure that you have gone through the previous article on Resistance of Conductor.
We have learnt-
- Resistance of a conductor is the property by virtue of which it opposes the motion of charge carriers through it.
- The collisions of free electrons with the positive metal ions is the basic cause of resistance.
In this article, we will discuss the temperature dependence of resistance for-
- Metals
- Alloys
- Semiconductors & Insulators
- Electrolytes
Temperature Dependence of Resistance-
For Metals (Conductors)-
- As temperature increases, the thermal speed of the free electrons increases and also the amplitude of vibration of the metal ions increases.
- Consequently, the free electrons collide more frequently with the metal ions.
- As a result, the path of free electrons is obstructed which are responsible for conductivity.
Hence,
|
On increasing the temperature,
the resistance of metals (conductors) increases. |
For most of the metals, resistance increases linearly with the rise in temperature.
In such cases, resistance at any temperature T (>T0) is given by-
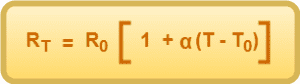
where-
- RT = Resistance at temperature T
- R0 = Resistance at a reference temperature T0
- α = Temperature coefficient of resistance
For Alloys-
|
On increasing the temperature,
the resistance of alloys increases but the increase is not much. |
It is interesting to note that-
For some metal alloys, the temperature coefficient of resistance is very close to zero meaning that the resistance hardly changes at all with the temperature.
Why alloys like constantan or manganin are used for making standard resistors?
It is because of the following reasons-
|
For Semiconductors and Insulators-
Semiconductors and insulators typically have negative temperature coefficient of resistance meaning that-
|
On increasing the temperature,
the resistance of semiconductors and insulators decreases. |
For insulators, on increasing the temperature, resistance decreases because of the increase in number of free electrons.
For semiconductors, on increasing the temperature, resistance first decreases (upto 400 K) due to generation of more number of holes and electron pairs, then remains constant (400 K to 600 K) but after 600 K, it increases due to the increased number of collisions.
![]()
For Electrolytes-
As the temperature increases, the inter-ionic attractions (solute-solute, solvent-solute and solvent-solvent types) decreases and also the viscous forces decreases and therefore the ions are able to move more freely.
Hence,
|
As the temperature of electrolytic solution increases,
the resistance of electrolyte decreases. |
Test Your Concepts-
Quiz on Temperature Dependence of Resistance
Next Article-
Get more notes & other study material of the Chapter Current Electricity.

